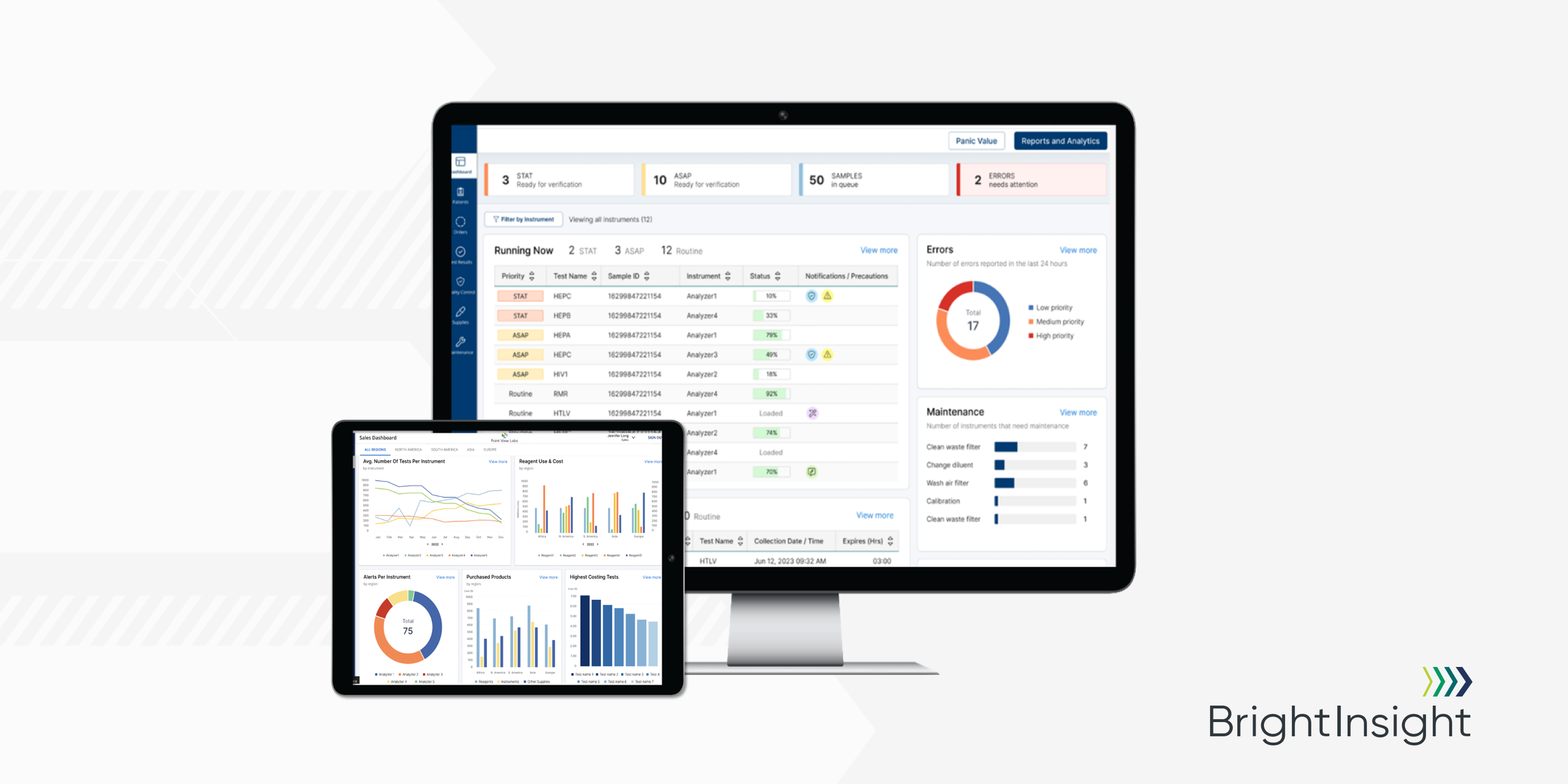
This week at the 2022 AACC Annual Scientific Meeting and Clinical Lab Expo in Chicago, I am honored to announce the launch of BrightInsight’s new Connected Diagnostics Platform.
This comprehensive end-to-end platform is the latest BrightInsight product launch designed to help diagnostic companies unlock opportunities in regulated digital health to harness Real-World Data, accelerate time to market and optimize workflows.
Following on the heels of our recent Disease Management Solution launch, our Connected Diagnostics Platform brings digital transformation to in vitro diagnostics (IVD). It’s estimated that 60 to 70 percent of healthcare decisions are influenced by IVD test results, so it’s critical for IVD manufacturers to manage their devices and data securely and reliably. This platform is designed to meet the demands of IVD manufacturers, helping them take advantage of the growth that’s projected in the market over the next decade—it’s projected to grow about 50 percent, from $71 billion in 2022 to $107 billion by 2029.
Despite being poised for growth, many medtech companies are behind the digital curve. Across the board, medtech companies have told us they’re investing in device connectivity, yet many struggle with a wide range of challenges, including:
Our Connected Diagnostics Platform is a one-stop solution for in vitro diagnostics manufacturers, enabling them to streamline workflows, accelerate revenue growth and drive innovation.
The platform is actually four powerful tools in one, featuring a Proxy Agent, Analytics Dashboards, Integration Middleware and Workflow Portals for the IVD manufacturer and the lab. Of course, all these tools run on the compliant and secure BrightInsight Platform.
Through this launch, we’re helping medtech companies eliminate the need for multiple products and partners, accelerate time to market, leverage best-in-class cybersecurity to reduce risk and position themselves for future growth and scalability.
The Connected Diagnostics Platform turns Real-World Data into actionable insights about users, devices, workflows and clinical decision making. Leading medtech manufacturers will have all the information they need to unlock innovation, while also being able to deliver consistent clinical workflows to their customers.
This launch is just the latest example of how BrightInsight is pursuing our mission of transforming patient outcomes globally through the power of digital technology. It’s an exciting time of growth for us, one that is piloted by both the innovative minds who work at BrightInsight and the healthcare, technology and business thought leaders who advise us. I’d like to welcome our newest BrightInsight Advisory Council member, Stephen N. Oesterle, M.D., a renowned expert in digital and medtech who sums up this momentous period of innovation
Want to learn more about how our Connected Diagnostic Platform is transforming IVD? Contact us or request a demo.